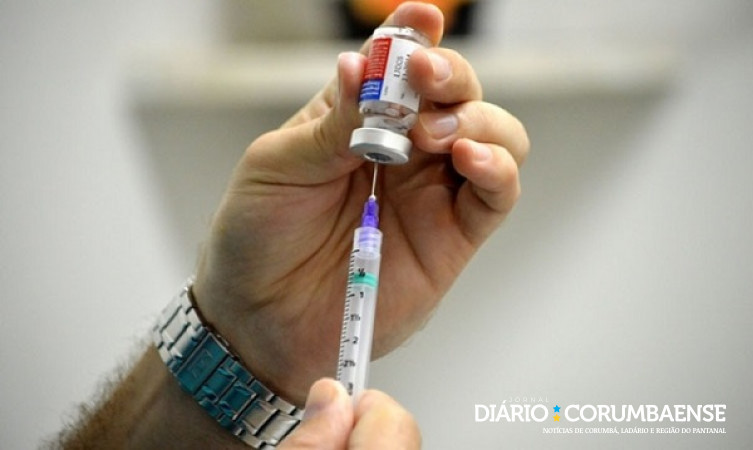
Pfizer: Countries like the US, EU members, Canada, Chile and others have been vaccinating children aged 5 and over since November (© Paul Hennessy / SOPA Images / Sipa USA / Agência Brasil)
Medicines Regulatory Agency in United kingdom This Wednesday approved the use of a vaccine from Pfizer With BioNTech against covid-19 for Children from 5 to 11 years oldAfter the agency determines that the dose is safe and effective.
The country’s Vaccine Committee has advised that children in an age group who are in the clinical risk group should receive the vaccine.
Children will receive two 10 microgram doses of the Pfizer-BioNTech vaccine – one-third of the dose for adults – with an eight-week gap between the first and second doses, the British Joint Committee on Vaccination and Immunization said.
Countries such as the US, EU members, Canada, Chile and others have vaccinated 5-year-olds since November. In the United States, for example, 7,141,428 doses of Pfizer’s pediatric vaccine have been given to children ages 5 to 11, as of December 9. Of these, 5,126,642 received the first dose and 2,014,786 received the second dose.
Vaccine for children in Brazil
The vaccination of children and adolescents from 5 to 11 years of age in Brazil has been approved by the National Health Surveillance Agency (infinite) last Thursday, the 16th, when the local authority allowed Pfizer to add this age group to its COVID-19 vaccine package.
Children from 5 to 11 years old continue to be vaccinated without a specific deadline despite their release. Anfisa’s decision faces opposition from the federal government, which has criticized the measure. Anvisa servers have also suffered from threats and retaliation on social networks. This week, boss Jair Bolsonaro He returned to criticize the approval, saying that Anfisa’s decision was “unbelievable”.
After the deadlock, Minister Ricardo Lewandowski, of the Federal Supreme Court, was Two days were given to the government to demonstrate. After that, it extended the deadline, setting a deadline of January 5 for the Ministry of Health to submit a schedule for immunizing children.
Also this week, the Oswaldo Cruz Foundation A study was published stating that vaccination of children will be critical for Brazil to achieve herd immunity. The country has reached 66% of the population fully vaccinated against COVID-19, but the progress of the Micron variant is worrisome.
Last Tuesday, 21, the agency asked the Butantan Institute for more information to analyze the consent to use andFusion Coronavac in the children’s audience. The decision was made after a meeting with technicians from Anvisa, representatives of the Butantan Institute and medical societies.

“Friendly zombie guru. Avid pop culture scholar. Freelance travel geek. Wannabe troublemaker. Coffee specialist.”